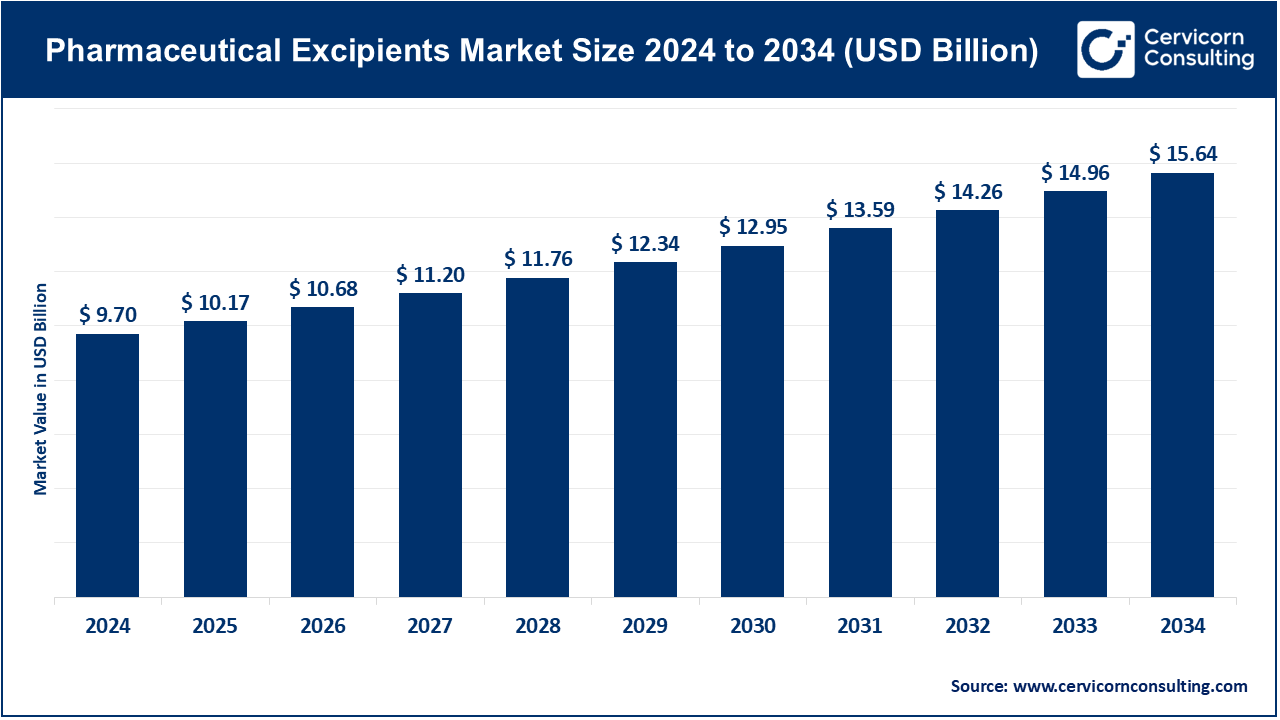
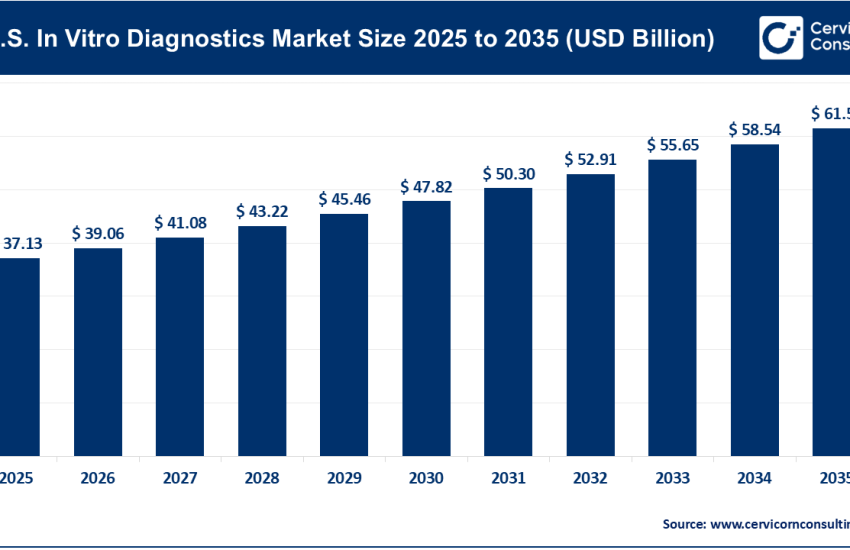
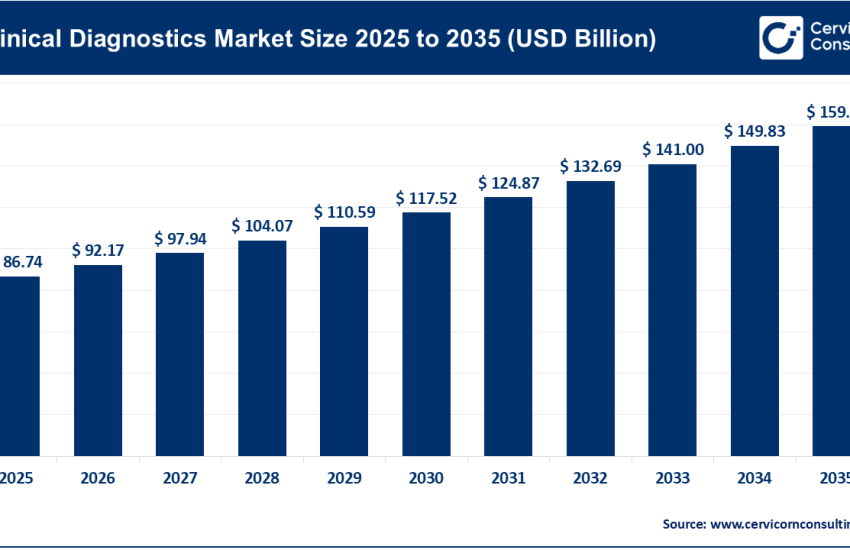
1. Growing Demand for Multifunctional Excipients
Pharmaceutical formulations increasingly require multifunctional excipients capable of serving multiple roles. Beyond acting as traditional binders or fillers, these excipients enhance solubility, improve bioavailability, and enable targeted release mechanisms. For instance, excipients like Polyplasdone Plus streamline manufacturing by improving tablet disintegration while addressing specific patient needs.
2. Transition to Natural and Bio-Based Excipients
Driven by rising awareness of safety and environmental sustainability, pharmaceutical companies are favoring natural and bio-based excipients. Plant-derived substances, such as starch and cellulose, are gaining traction due to their formulation compatibility and perceived safety over synthetic alternatives. Regulatory encouragement for safer materials further supports this trend.
3. Innovations in Drug Delivery Technologies
Cutting-edge technologies like 3D printing and continuous manufacturing are revolutionizing drug delivery systems. These advancements enable the creation of complex formulations tailored to patient needs, including personalized medicine. As a result, excipients are evolving from passive components to critical elements designed to enhance functionality in these innovative delivery systems.
4. Emphasis on Personalized Medicine
The rise of personalized medicine is spurring the development of specialized excipients for specific patient demographics, such as pediatric and geriatric populations. These formulations often include unique features like taste masking or ease of swallowing. Consequently, pharmaceutical companies are investing more in research to develop novel, tailored excipients.
5. Heightened Regulatory Focus
Regulatory bodies are tightening guidelines to prioritize excipients with proven safety profiles. Manufacturers are increasingly required to comply with strict safety and efficacy standards. This growing emphasis on compliance is driving innovation in excipient development to meet regulatory expectations.
6. Expansion of Emerging Markets and Generic Drug Demand
The growth of pharmaceutical production in emerging markets, particularly in Asia-Pacific and Latin America, is fueling demand for excipients. These regions are experiencing a surge in generic drug manufacturing, driven by the prevalence of chronic diseases and the need for innovative formulations to improve treatment outcomes and patient adherence.
7. Integration of Artificial Intelligence in Development
Artificial intelligence (AI) and predictive modeling are becoming integral to the excipient development process. These technologies enable manufacturers to optimize formulations by forecasting the effects of excipient variations on drug performance, improving the efficiency of development cycles and reducing time-to-market.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2453