Full-Service CRO Market Trends, Growth Drivers, and Global Outlook 2024–2034
Full-Service CRO Market Size and Growth Factor
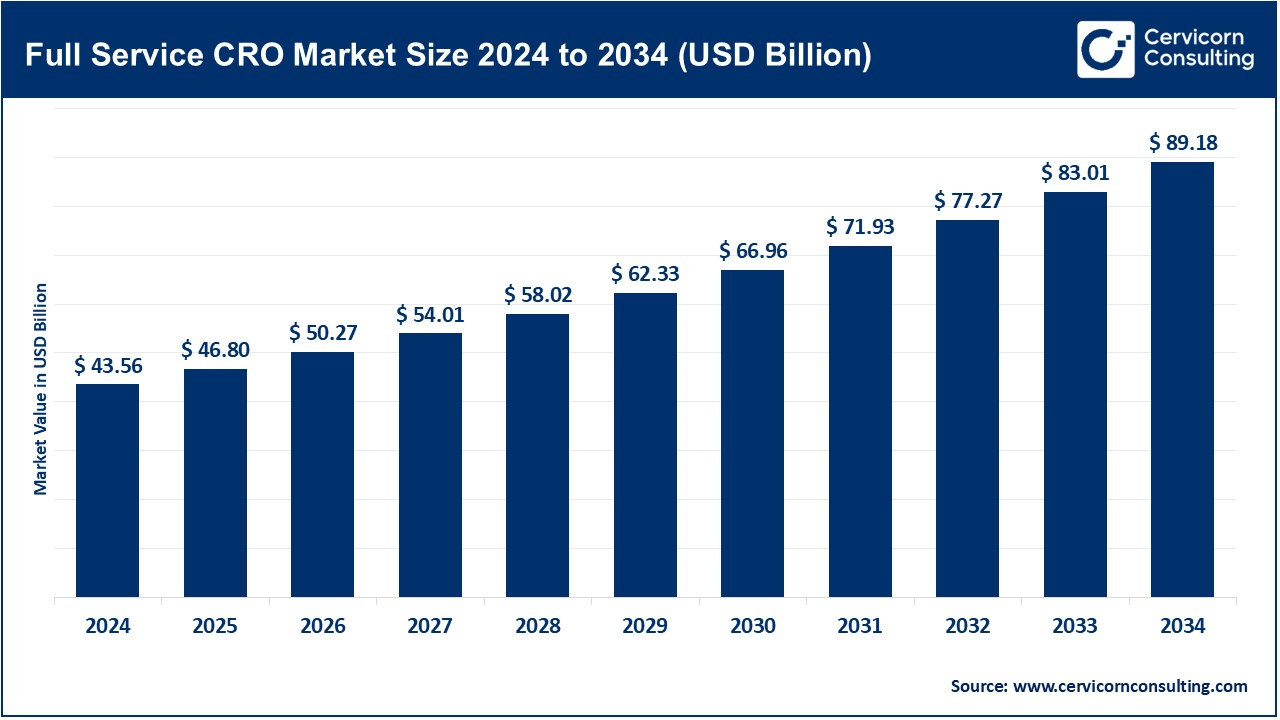
The global full-service CRO market was worth USD 43.56 billion in 2024 and is anticipated to expand to around USD 89.18 billion by 2034, registering a compound annual growth rate (CAGR) of 7.42% from 2025 to 2034.
The full-service Contract Research Organization (CRO) market is experiencing significant growth, driven by factors such as the increasing complexity of drug development, the need for cost-effective and efficient clinical trials, and the rising demand for comprehensive services that span the entire drug development lifecycle. Biopharmaceutical companies are increasingly outsourcing to full-service CROs to streamline processes, reduce timelines, and access specialized expertise, thereby accelerating the development and approval of new therapies.
What is the Full-Service CRO Market?
A full-service CRO offers end-to-end services encompassing all phases of clinical research, from preclinical studies to post-marketing surveillance. These services include regulatory affairs, site selection and activation, patient recruitment, data management, biostatistics, pharmacovigilance, and medical writing. By providing a comprehensive suite of services under one roof, full-service CROs enable pharmaceutical and biotechnology companies to conduct clinical trials more efficiently and effectively.
Why is it Important?
The importance of full-service CROs lies in their ability to manage the increasing complexity of clinical trials, which often require specialized knowledge, global reach, and adherence to stringent regulatory standards. By partnering with full-service CROs, sponsors can leverage their expertise to navigate regulatory hurdles, access diverse patient populations, and implement innovative technologies, ultimately accelerating the development of new therapies and bringing them to market more quickly.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2615
Top Companies in the Full-Service CRO Market
Medpace
- Specialization: Full-service clinical development for biopharmaceutical and medical device companies.
- Key Focus Areas: Cardiology, oncology, endocrinology, and infectious diseases.
- Notable Features: Integrated approach with in-house regulatory, clinical operations, and laboratory services.
- Global Presence: Operations in over 40 countries.
Laboratory Corporation of America Holdings (Labcorp)
- Specialization: Comprehensive laboratory services and drug development support.
- Key Focus Areas: Oncology, cardiovascular, and infectious diseases.
- Notable Features: Extensive laboratory network and expertise in diagnostics.
- Global Presence: Operations in over 100 countries.
ICON plc
- Specialization: Clinical development and commercialization services.
- Key Focus Areas: Oncology, CNS, and rare diseases.
- Notable Features: Acquisition of PRA Health Sciences to enhance service offerings.
- Global Presence: Operations in over 55 countries.
IQVIA Inc
- Specialization: Healthcare data analytics and clinical research services.
- Key Focus Areas: Real-world evidence, patient-centered trials, and digital health.
- Notable Features: Integration of advanced analytics and technology in clinical trials.
- 2024 Revenue (approx.): $15.43 billion to $15.53 billion.
- Global Presence: Operations in over 100 countries.
Syneos Health
- Specialization: Biopharmaceutical solutions and integrated clinical development.
- Key Focus Areas: Neurology, oncology, and immunology.
- Notable Features: Combines clinical development with commercialization services.
- Global Presence: Operations in over 110 countries.
Leading Trends and Their Impact
The full-service CRO market is influenced by several key trends:
- Decentralized Clinical Trials (DCTs): The adoption of DCTs allows for remote patient monitoring and data collection, enhancing patient engagement and reducing trial costs.
- Integration of Artificial Intelligence (AI): AI is being used to optimize trial design, patient recruitment, and data analysis, leading to more efficient and accurate outcomes.
- Emphasis on Real-World Evidence (RWE): RWE is increasingly utilized to support regulatory decisions and post-market surveillance, providing insights into treatment effectiveness in real-world settings.
- Focus on Patient-Centric Approaches: Engaging patients throughout the trial process improves retention rates and ensures that studies are more aligned with patient needs.
Successful Examples Around the World
- Asia-Pacific Region: The APAC region, particularly countries like China and India, has seen significant growth in the CRO market due to cost advantages, large patient populations, and favorable regulatory environments.
- North America: The U.S. remains a leader in clinical trials, supported by a robust regulatory framework and advanced healthcare infrastructure.
Regional Analysis Including Government Initiatives and Policies
- Asia-Pacific: Governments in the APAC region are implementing policies to attract clinical research, such as streamlined approval processes and investment in healthcare infrastructure.
- North America: The U.S. Food and Drug Administration (FDA) provides guidance to facilitate clinical trials, including support for innovative trial designs and patient-focused drug development.
- Europe: The European Medicines Agency (EMA) promotes harmonization of clinical trial regulations across member states, aiming to create a more efficient and collaborative research environment.
These regional initiatives contribute to the dynamic growth and evolution of the full-service CRO market, enabling more efficient and patient-centered clinical research globally.
To Get Detailed Overview, Contact Us: https://www.cervicornconsulting.com/contact-us
Read Report: Pharmaceutical Data and Analytics Market Size Worth $4.18 Billion by 2034: Key Trends & Insights