CRISPR Gene Editing Market Revenue, Global Presence, and Strategic Insights by 2034
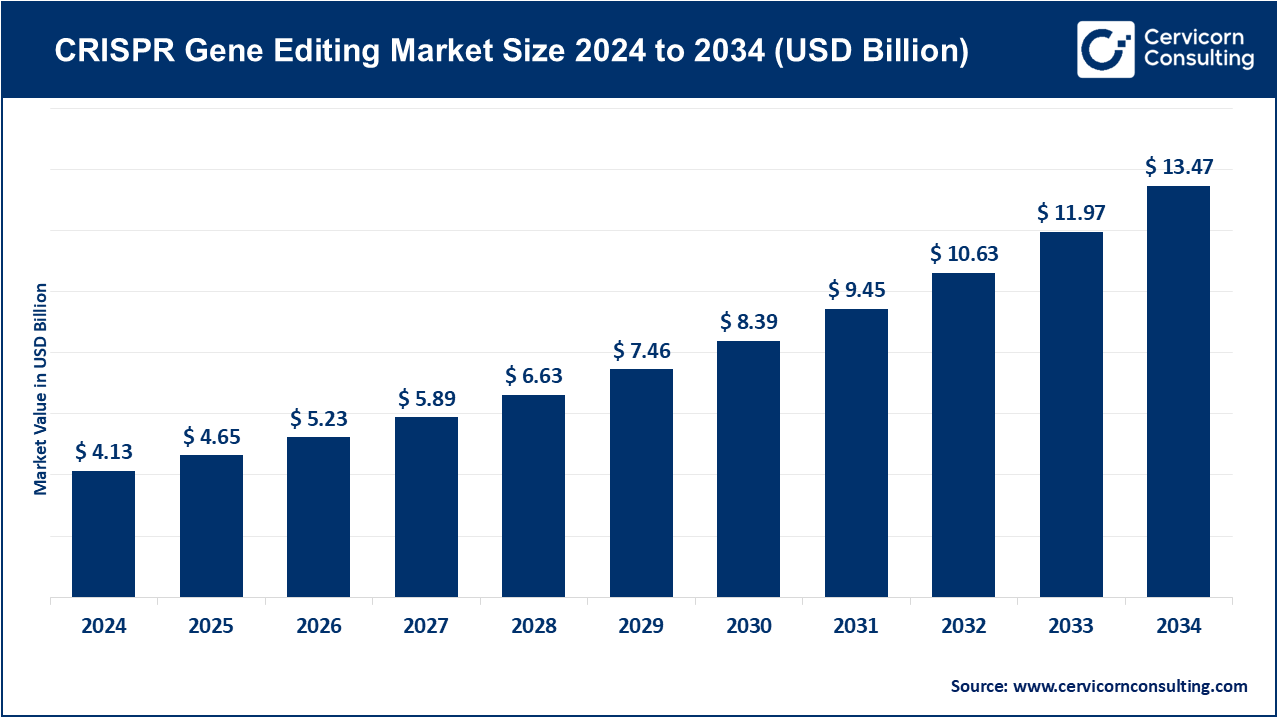
CRISPR Gene Editing Market Size
CRISPR Gene Editing Market Growth Factors
The CRISPR gene editing market is propelled by several converging factors: rapid advancements in genome-editing technologies that enhance accuracy and minimize off-target effects; expanding clinical pipelines targeting both rare monogenic and complex diseases; surging R&D investments from biotechnology firms, pharmaceutical giants, and venture capital funds; continuous innovation in base and prime editing tools that broaden therapeutic scope; increasing adoption of CRISPR systems in agriculture, biotechnology, and research; and supportive government initiatives promoting precision medicine and gene therapy research. Additionally, partnerships and mergers between established life-science companies and emerging biotechs are accelerating commercialization, while global demand for personalized medicine and regenerative therapies drives adoption of CRISPR solutions across multiple industries.
What is the CRISPR Gene Editing Market?
The CRISPR gene editing market encompasses all commercial activities revolving around Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) proteins, such as Cas9 and Cas12. It includes the development, production, and sale of gene-editing tools, reagents, and consumables used in basic and applied research, along with therapeutics in clinical pipelines, agricultural applications, and industrial biotechnology. The market spans reagents, plasmids, enzymes, kits, instruments, delivery systems, and bioinformatics tools that assist in designing and executing gene edits. It also covers contract research and manufacturing services, clinical trial operations, and regulatory support systems for gene-edited therapies. In essence, it represents the ecosystem supporting research, development, and commercialization of genome editing for medical, agricultural, and industrial uses.
Why is CRISPR Gene Editing Important?
CRISPR technology has transformed life sciences by enabling precise, efficient, and cost-effective DNA modification. Its ability to target specific genes and edit them with high accuracy allows scientists to correct genetic defects, develop resistant crop varieties, and engineer microorganisms for bio-industrial production. In healthcare, CRISPR offers a potential cure for genetic diseases such as sickle cell anemia, beta-thalassemia, and cystic fibrosis.
In agriculture, it enables the creation of crops with enhanced yields, drought resistance, and nutritional value without introducing foreign DNA—offering a more acceptable form of genetic improvement. Industrially, CRISPR accelerates strain engineering for pharmaceuticals, enzymes, and biofuels. The technology’s modular nature and low cost democratize genome editing, making it accessible to academic labs, biotech startups, and pharmaceutical companies alike. Ultimately, CRISPR is vital for transitioning from traditional therapies and crop breeding methods toward more precise, efficient, and sustainable biological solutions.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2773
CRISPR Gene Editing Market Top Companies
Below is a detailed comparison of key players shaping the CRISPR gene editing market, highlighting their specialization, focus areas, distinctive features, revenues, and global presence.
1. Thermo Fisher Scientific
- Specialization: Life-science tools, reagents, instruments, and services supporting gene-editing research and clinical development.
- Key Focus Areas: Reagents for molecular biology, genome editing kits, sequencing instruments, and bioprocessing solutions used in gene therapy manufacturing.
- Notable Features: A vertically integrated portfolio combining reagents, hardware, software, and manufacturing services. Its supply chain strength and global distribution network make it indispensable to laboratories and biotech firms working with CRISPR.
- 2024 Revenue: Approximately USD 42.88 billion.
- Market Share: One of the largest enablers of CRISPR workflows through reagent and equipment supply, though not a direct therapeutic developer.
- Global Presence: Strong distribution network across North America, Europe, Asia-Pacific, and emerging markets, with thousands of institutional customers.
2. Merck KGaA (MilliporeSigma)
- Specialization: Advanced life-science reagents and materials for gene editing, cell culture, and bioprocessing.
- Key Focus Areas: CRISPR reagents and kits for research, customized genome-editing services, and tools for gene and cell therapy development.
- Notable Features: Pioneer in the CRISPR research reagent space; robust IP portfolio and extensive collaborations with academic institutions.
- 2024 Revenue: Approximately EUR 21.2 billion.
- Market Share: A leading European provider of CRISPR reagents and cell-line engineering tools.
- Global Presence: Strong foothold in Europe, North America, and Asia; operates major R&D and production facilities globally.
3. Integrated DNA Technologies (IDT)
- Specialization: Synthetic DNA and RNA oligonucleotides, including custom CRISPR guide RNAs and donor templates.
- Key Focus Areas: High-fidelity oligonucleotide synthesis, reagent kits for CRISPR experiments, and computational tools for guide RNA design.
- Notable Features: Recognized globally for precision, scalability, and reliability in oligonucleotide synthesis; integral supplier for CRISPR reagents.
- 2024 Revenue: Part of Danaher Corporation (segment revenue estimated in the billions; IDT contributes several hundred million USD annually).
- Market Share: Dominant player in CRISPR guide and oligonucleotide reagents for academic and commercial research.
- Global Presence: Operations in North America, Europe, and Asia through Danaher’s extensive life-sciences network.
4. GenScript Biotech
- Specialization: Gene synthesis, protein engineering, and CRISPR screening libraries with a strong service orientation.
- Key Focus Areas: Custom gene synthesis, CRISPR libraries for functional genomics, and contract research and manufacturing services for biopharma clients.
- Notable Features: Leading provider of synthetic biology services in Asia; integrates gene editing with antibody and cell-line development capabilities.
- 2024 Revenue: Approximately USD 594.5 million (continuing operations).
- Market Share: Prominent in Asia-Pacific’s CRISPR reagents and services market, serving both academic and industrial clients.
- Global Presence: Headquarters in Nanjing, China with subsidiaries and logistics hubs in the U.S., Europe, and Japan.
5. Takara Bio
- Specialization: Molecular biology reagents, cloning kits, and gene-editing tools for research and clinical development.
- Key Focus Areas: CRISPR/Cas9-based genome editing kits, cell culture reagents, and advanced tools for gene and cell therapy research.
- Notable Features: Long-standing reputation in molecular biology innovation; active in developing enabling technologies for regenerative medicine and genetic engineering.
- 2024 Revenue: Reported through fiscal year 2024 statements (Japanese yen figures converted to hundreds of millions USD).
- Market Share: Well-established supplier in Asia, expanding into global research reagent markets.
- Global Presence: Headquarters in Japan with subsidiaries across the U.S., Europe, and Asia-Pacific.
Leading Trends and Their Impact
- Clinical Translation and Therapeutic Breakthroughs:
The most transformative trend is the clinical success of CRISPR-based therapies for blood disorders like sickle cell disease and beta-thalassemia. As these therapies gain regulatory approval, investor confidence and large-scale manufacturing partnerships surge. The ripple effect includes new programs targeting eye diseases, cancers, and liver disorders, positioning CRISPR therapeutics as a new medical frontier. - Emergence of Next-Generation Editors:
Base editing and prime editing technologies are revolutionizing precision gene correction. These tools avoid double-strand breaks, reducing the risk of off-target mutations and expanding CRISPR’s usability in sensitive tissues and in vivo applications. Their commercialization will likely spawn specialized reagent and delivery markets. - Improved Delivery Mechanisms:
Advances in lipid nanoparticles, viral vectors, and non-viral carriers enhance CRISPR delivery efficiency. Successful in vivo delivery remains a key challenge, and innovations here determine the scalability of CRISPR therapies. Firms focusing on delivery optimization will capture significant market share in the coming years. - CRISPR Beyond Human Therapeutics:
Industrial and agricultural CRISPR applications are broadening the technology’s market scope. In crops, CRISPR accelerates the creation of climate-resilient and high-yield varieties without introducing foreign genes, allowing smoother regulatory acceptance. In industrial biotech, CRISPR shortens strain-engineering timelines for producing enzymes, specialty chemicals, and biofuels. - Ethical Oversight and Regulatory Governance:
The growth of CRISPR has spurred governments to refine bioethics and biosafety policies. Global authorities now demand extensive off-target testing, genomic surveillance, and long-term patient monitoring. While this increases compliance costs, it also builds public trust and investor confidence. - Consolidation and Strategic Alliances:
Large life-science conglomerates are acquiring specialized CRISPR tool developers and forming partnerships with therapeutics startups. This trend enhances end-to-end service capabilities—from discovery to GMP manufacturing—but may also increase entry barriers for smaller players.
Successful CRISPR Applications Around the World
- Sickle Cell Disease and Beta-Thalassemia:
CRISPR-edited hematopoietic stem cell therapies have shown remarkable success in clinical trials, enabling patients to produce healthy red blood cells and significantly reducing disease symptoms. These milestones have demonstrated real-world therapeutic viability and attracted major biopharma investments. - Inherited Retinal Disorders:
In vivo CRISPR therapies targeting mutations that cause blindness have achieved successful early-stage trial outcomes, proving that gene editing can work safely within the human body. This paves the way for ocular and neurological applications. - Agricultural Innovation:
CRISPR has been applied to develop rice, maize, and tomato varieties with enhanced nutritional profiles, disease resistance, and longer shelf life. These advancements address food security challenges and support sustainable agriculture without relying on transgenic methods. - Industrial Biotechnology:
CRISPR is used to engineer microbial strains for faster, more efficient production of enzymes, pharmaceuticals, and renewable biofuels. Such developments shorten R&D timelines, improve yields, and reduce industrial waste.
These successful implementations across sectors confirm CRISPR’s versatility and growing commercial maturity, reinforcing its importance in medicine, agriculture, and sustainable manufacturing.
Global Regional Analysis
North America
North America holds the dominant market share due to its strong biotech infrastructure, significant venture capital funding, and a large number of clinical trials. The U.S. government actively supports CRISPR research through NIH and DoD programs, and the FDA has implemented adaptive pathways for gene therapy approvals. Canada complements this ecosystem with robust academic research and favorable regulations for genomics startups. Together, these factors establish North America as the innovation and commercialization hub for CRISPR technologies.
Europe
Europe represents a mature and regulated market characterized by strong academic collaboration and industrial participation. The European Union funds genome-editing research through Horizon Europe and other programs, promoting responsible innovation. The UK, Germany, France, and Switzerland are particularly active in CRISPR-based biotech development. Ethical oversight is stringent, focusing on ensuring patient safety and environmental sustainability. Despite slower regulatory approval processes, Europe’s stable funding and scientific rigor ensure steady growth.
Asia-Pacific
Asia-Pacific is the fastest-growing region in the CRISPR market. China leads in patent filings, agricultural CRISPR projects, and clinical trial volume, supported by state-backed biotechnology initiatives. Japan and South Korea have robust pharmaceutical industries integrating CRISPR into regenerative medicine and immunotherapy. India and Australia are emerging players focusing on academic research and collaborative projects. Government investments in biotech infrastructure and supportive regulatory reforms are accelerating commercialization throughout the region.
Latin America
Latin America’s CRISPR market is still emerging but shows promise, especially in agricultural and public-health applications. Brazil, Argentina, and Mexico are investing in biotech research to enhance crop productivity and address local disease burdens. Partnerships with international research institutions and access to CRISPR reagent suppliers are fostering gradual market penetration.
Middle East & Africa
This region remains in the early stages of CRISPR adoption, with growing academic interest in genetic and agricultural research. Countries like Israel and the United Arab Emirates are exploring partnerships with global biotech firms to build local genomics capacity. Government policies emphasizing health innovation and food security may drive future expansion.
Government Initiatives and Policies Shaping the Market
- Regulatory Modernization:
Governments worldwide are updating regulatory frameworks to accommodate genome-editing therapies. Agencies like the FDA, EMA, and PMDA have issued guidance on safety testing, manufacturing quality, and long-term monitoring. - Ethical and Social Governance:
International organizations and national bioethics councils are establishing boundaries on germline editing while encouraging therapeutic and agricultural research that aligns with public interest. - Public Funding Programs:
Large-scale funding initiatives in the U.S., EU, Japan, and China support academic-industrial collaboration in genome editing, emphasizing therapeutic innovation and biomanufacturing infrastructure. - Education and Workforce Development:
Governments and universities are investing in genomics education to prepare skilled professionals for the rapidly evolving biotech industry, ensuring a capable workforce to sustain growth. - Global Collaboration:
Cross-border partnerships facilitate knowledge exchange, harmonize safety standards, and promote equitable access to CRISPR-based healthcare innovations.
These policies collectively balance innovation with responsibility, supporting CRISPR’s expansion while addressing ethical and safety considerations.
Key Insights for Stakeholders
- Researchers and Academics:
Continued demand for high-precision reagents, reliable bioinformatics platforms, and improved off-target detection tools will define research needs. - Biotech and Pharmaceutical Firms:
Strategic collaborations with reagent suppliers, contract manufacturers, and data-analytics firms are crucial for accelerating clinical translation. - Investors and Venture Capitalists:
Opportunities lie in next-generation editors, delivery technologies, and service providers offering GMP-grade CRISPR production. - Policy Makers and Regulators:
The challenge is to foster innovation while maintaining public trust, particularly for in vivo editing and agricultural applications.
To Get Detailed Overview, Contact Us: https://www.cervicornconsulting.com/contact-us
Read Report: Telehealth 2.0 Market Revenue, Global Presence, and Strategic Insights by 2034