CAR T-Cell Therapy Market Growth, Key Trends, and Leading Companies (2025-2034)
Table of Contents
ToggleCAR T-cell Therapy Market Overview
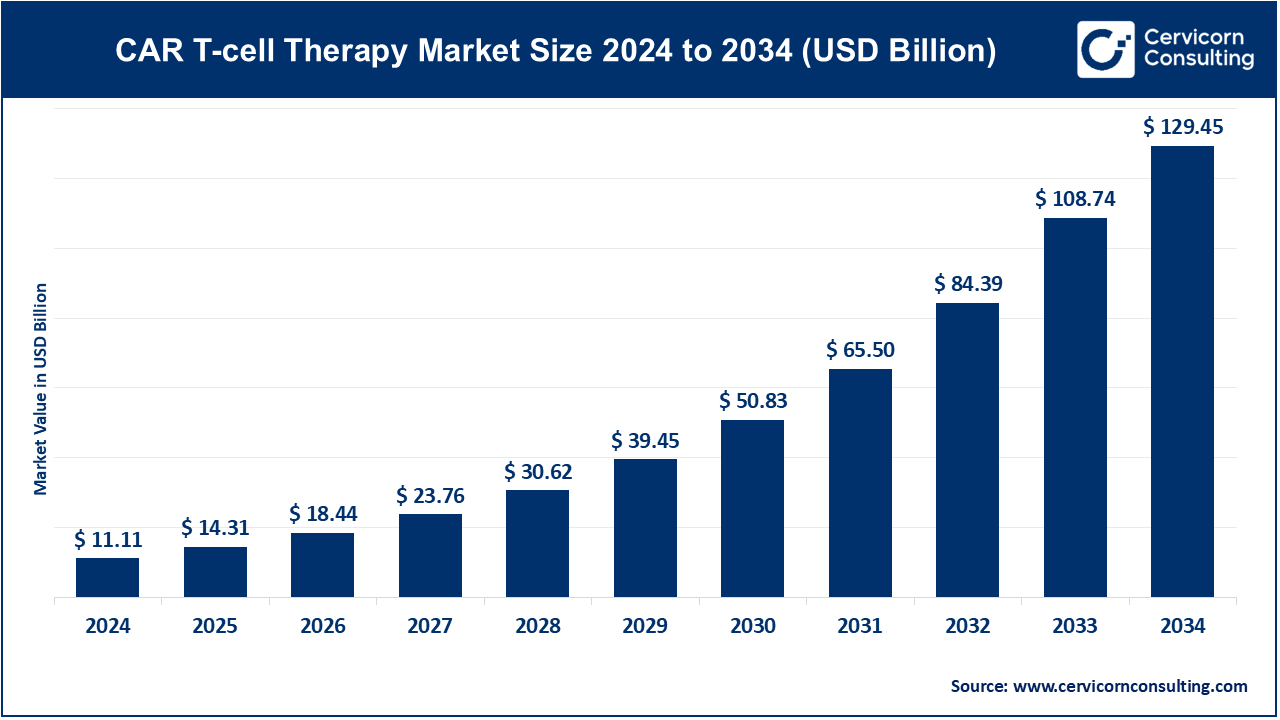
The global CAR T-cell therapy market was valued at USD 11.11 billion in 2024 and is projected to grow to approximately USD 129.45 billion by 2034, with a compound annual growth rate (CAGR) of 27.83% from 2025 to 2034.
The CAR T-cell therapy market has experienced robust growth in recent years, driven by advancements in immuno-oncology, the increasing prevalence of cancer, and the growing demand for personalized treatment options. Rising investments in R&D, expanding healthcare infrastructure, and increasing healthcare budgets worldwide have significantly contributed to the rapid market expansion.
Furthermore, growing awareness of the benefits of CAR T-cell therapy among both healthcare professionals and patients, combined with the advent of more efficient manufacturing processes and the broadening of CAR T-cell therapies beyond hematological malignancies to solid tumors, is fueling market momentum. Strategic mergers and acquisitions among key players also play a critical role in enhancing innovation and access to global markets.
What is CAR T-cell Therapy Market?
Chimeric Antigen Receptor (CAR) T-cell therapy is an innovative form of immunotherapy that involves the genetic modification of a patient’s T-cells to express receptors specific to cancer cells. These engineered T-cells are then reintroduced into the patient’s body to target and destroy malignant cells, particularly those associated with blood cancers like lymphoma, leukemia, and multiple myeloma. CAR T-cell therapy has revolutionized cancer treatment by providing a personalized and highly effective treatment option, especially in cases where traditional therapies, such as chemotherapy or radiation, have failed. This market includes the companies, biopharmaceuticals, research institutions, and healthcare providers that are involved in developing, manufacturing, and distributing CAR T-cell therapies.
Why is it Important?
CAR T-cell therapy represents a transformative shift in the oncology landscape by offering a targeted, highly effective treatment for cancers that were once considered incurable. Unlike conventional cancer treatments that affect both healthy and malignant cells, CAR T-cell therapy specifically targets cancer cells, significantly reducing side effects and improving patient outcomes. Moreover, CAR T-cell therapy has demonstrated remarkable efficacy in treating hematologic cancers such as acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma, and other blood-related cancers, providing hope for patients with limited treatment options. The importance of CAR T-cell therapy extends beyond just treatment effectiveness; it also highlights the ongoing progress in genetic engineering, cell therapy, and personalized medicine, shaping the future of cancer care.
Key Companies in the Global CAR T-cell Therapy Market
1. Gilead Sciences, Inc.
Specialization: Immuno-oncology, Biotechnology
Key Focus Areas: Oncology, Immunotherapy, CAR T-cell Therapy
Notable Features: Gilead’s Kite Pharma is a leader in CAR T-cell therapy, particularly known for Yescarta, a treatment for certain types of lymphoma.
2024 Revenue: $30 billion (approx.)
Market Share: ~25% (approx.)
Global Presence: Gilead operates in over 100 countries with a strong presence in North America, Europe, and Asia-Pacific.
2. Novartis International AG
Specialization: Pharmaceuticals, Immuno-oncology
Key Focus Areas: Oncology, CAR T-cell Therapy, Gene Therapy
Notable Features: Novartis’ Kymriah was the first CAR T-cell therapy approved by the FDA for certain types of leukemia and lymphoma.
2024 Revenue: $53 billion (approx.)
Market Share: ~30% (approx.)
Global Presence: Novartis has a significant presence globally, with operations in more than 140 countries.
3. Bristol-Myers Squibb Company
Specialization: Biopharmaceuticals, Oncology, Immuno-oncology
Key Focus Areas: Oncology, CAR T-cell Therapy, Immuno-oncology
Notable Features: Bristol-Myers Squibb is a key player in CAR T-cell therapy with its approval of Breyanzi for lymphoma treatment.
2024 Revenue: $47 billion (approx.)
Market Share: ~10% (approx.)
Global Presence: The company operates in over 70 countries, with a robust presence in North America, Europe, and emerging markets.
4. Celgene Corporation (Acquired by Bristol-Myers Squibb)
Specialization: Biopharmaceuticals, Immuno-oncology
Key Focus Areas: Oncology, CAR T-cell Therapy, Hematology
Notable Features: Celgene’s involvement in CAR T-cell therapy is centered around its investment in Juno Therapeutics, which focuses on cancer treatments.
2024 Revenue: Now consolidated under Bristol-Myers Squibb.
Market Share: N/A post-acquisition.
Global Presence: Celgene’s operations are integrated within Bristol-Myers Squibb’s global network.
5. Kite Pharma, Inc. (a Gilead company)
Specialization: Biotechnology, Immuno-oncology
Key Focus Areas: Oncology, CAR T-cell Therapy
Notable Features: Kite Pharma’s Yescarta has become a standard treatment for large B-cell lymphoma, expanding the scope of CAR T-cell therapy.
2024 Revenue: $3 billion (approx.)
Market Share: ~10% (approx.)
Global Presence: Global operations with a strong presence in the U.S., Europe, and Asia.
Leading Trends and Their Impact
The CAR T-cell therapy market is experiencing several notable trends that are shaping its future.
- Expansion into Solid Tumors: Initially, CAR T-cell therapies were largely focused on hematologic malignancies, but recent breakthroughs and clinical trials indicate promising results in solid tumors. This has the potential to significantly expand the market and provide new treatment options for cancers like breast, lung, and pancreatic cancers.
- Cost Reduction and Accessibility: CAR T-cell therapy has historically been expensive due to its complex manufacturing process. However, advances in cell engineering, automation, and scalability are leading to cost reductions, making therapies more accessible to patients worldwide.
- Personalized Medicine: As the field of CAR T-cell therapy progresses, more personalized treatment plans based on genetic profiling and specific tumor markers are being developed, offering higher efficacy and reduced side effects.
- Collaboration and Partnerships: Key players in the market are forming strategic partnerships with biotech companies, research institutions, and hospitals to accelerate the development and distribution of CAR T-cell therapies.
- Regulatory Advancements: Governments and regulatory bodies are increasingly providing faster approval pathways for CAR T-cell therapies, expediting the time it takes for new treatments to reach the market.
Successful Examples of CAR T-cell Therapy Around the World
- Yescarta by Kite Pharma: Yescarta has been a successful CAR T-cell therapy for adult patients with certain types of large B-cell lymphoma, offering a breakthrough for those who have not responded to other treatments.
- Kymriah by Novartis: Kymriah became the first FDA-approved CAR T-cell therapy, providing a life-saving treatment for children and young adults with relapsed or refractory acute lymphoblastic leukemia (ALL).
- Breyanzi by Bristol-Myers Squibb: Breyanzi is another successful CAR T-cell therapy that has shown effectiveness in treating large B-cell lymphoma and has been widely adopted by healthcare providers.
Regional Analysis and Government Initiatives Shaping the Market
North America
In North America, particularly the United States, CAR T-cell therapy has witnessed rapid adoption due to favorable regulatory frameworks, significant investments in biotechnology, and an advanced healthcare system. The FDA has accelerated approvals for CAR T-cell therapies, and the U.S. government supports research through various grants and funding initiatives. Insurance coverage, although improving, remains a challenge, with high treatment costs posing barriers for some patients. Still, the market is expected to continue growing due to the ongoing demand for novel cancer treatments and the presence of leading companies like Gilead Sciences and Novartis.
Europe
Europe also represents a significant market for CAR T-cell therapies. The European Medicines Agency (EMA) has been proactive in approving several CAR T-cell therapies, including Kymriah and Yescarta. European governments are increasingly incorporating these treatments into national healthcare systems, although cost remains a concern. The European market is expected to grow as healthcare infrastructure improves and regulatory hurdles continue to ease.
Asia-Pacific
The Asia-Pacific region is emerging as a high-growth area for CAR T-cell therapies. Countries like China and Japan are witnessing rapid advancements in CAR T-cell therapy, driven by rising cancer rates and expanding healthcare access. China, in particular, has seen a surge in domestic biotechnology companies focusing on CAR T-cell therapies. Government initiatives to modernize healthcare and foster innovation are further supporting market expansion. However, affordability remains a key challenge for broader adoption in certain areas of the region.
Latin America and Middle East & Africa
In Latin America and the Middle East & Africa, the adoption of CAR T-cell therapies is still in its early stages. While healthcare systems are evolving, the high cost of CAR T-cell therapies limits their accessibility. However, as regional governments invest more in healthcare infrastructure and innovative therapies, the market is expected to witness gradual growth. International collaborations and investments are also paving the way for greater access to CAR T-cell therapies in these regions.
Government Initiatives and Policies
Governments around the world are playing a critical role in shaping the CAR T-cell therapy market through supportive policies, regulations, and funding. In the U.S., initiatives like the Cancer Moonshot Program and various public-private partnerships have accelerated the development and availability of CAR T-cell therapies. In Europe, the European Commission’s Horizon 2020 program has provided funding for CAR T-cell research, and regulatory bodies such as the EMA have created pathways for quicker approval of innovative cancer therapies. Similarly, in Asia, countries like China have introduced supportive policies and incentives for biotechnology companies to invest in CAR T-cell therapies.
These government initiatives are crucial for fostering innovation, making therapies more accessible, and ensuring that CAR T-cell therapy becomes a cornerstone of cancer treatment globally.
To Get Detailed Overview, Contact Us: https://www.cervicornconsulting.com/contact-us
Read Report: Home Healthcare Market Growth Projections and Key Developments (2024-2033)