Pharma 4.0 Market 2024-2033: Growth, Trends, and Key Insights
Overview of the Pharma 4.0 Market
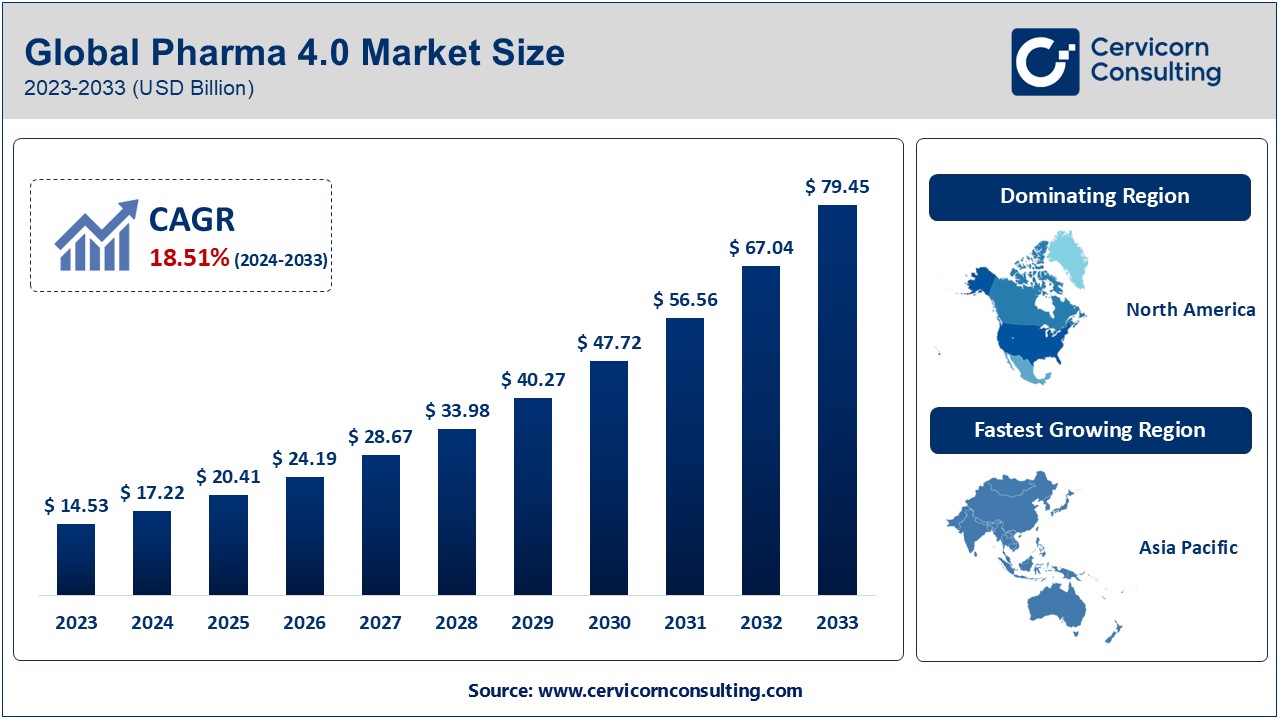
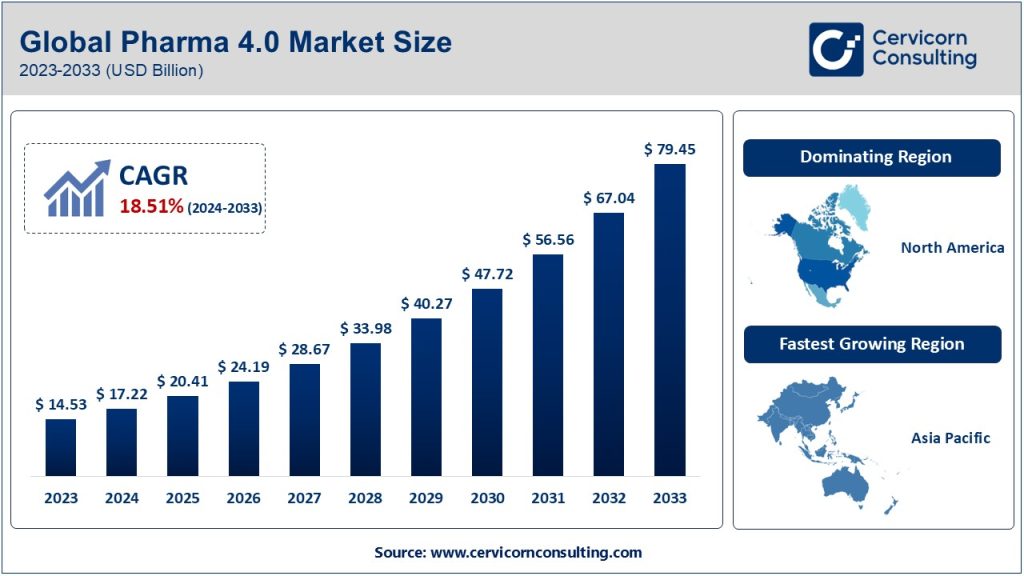
The Pharma 4.0 market is at the forefront of transforming the pharmaceutical and biopharmaceutical sectors, emphasizing automation, digitization, and advanced analytics. As of 2023, the market was valued at approximately USD 14.53 billion and is projected to reach USD 79.45 billion by 2033, exhibiting a CAGR of 18.1% during the forecast period from 2024 to 2033. This growth is propelled by the increasing adoption of smart technologies, regulatory demands for quality control, and the need for efficient production processes in the pharmaceutical industry.
What is Pharma 4.0?
Pharma 4.0 refers to the application of Industry 4.0 principles—such as IoT, AI, machine learning, and blockchain—to pharmaceutical manufacturing and operations. It aims to enhance productivity, improve compliance, and ensure product quality by enabling real-time decision-making and end-to-end connectivity.
How are regional government initiatives influencing the Pharma 4.0 market?
Government initiatives worldwide are playing a pivotal role in driving the adoption of Pharma 4.0 technologies. These initiatives focus on regulatory support, funding for innovation, and modernization of healthcare systems.
North America
- Regulatory Emphasis on Quality: The FDA’s Emerging Technology Program encourages pharmaceutical companies to adopt advanced manufacturing technologies, including continuous manufacturing and real-time analytics, to improve drug quality and reduce production costs.
- Incentives for Digital Transformation: Grants and tax incentives for pharmaceutical companies investing in digitalization are fostering a robust market for Pharma 4.0 solutions.
Europe
- Support for Smart Manufacturing: The European Medicines Agency (EMA) promotes digital innovation through frameworks like Good Automated Manufacturing Practice (GAMP 5), pushing companies to adopt Pharma 4.0 standards.
- Sustainability Goals: Initiatives such as the European Green Deal encourage the adoption of energy-efficient and sustainable manufacturing technologies in pharmaceutical production.
Asia-Pacific
- Policy-Driven Growth in China: Government programs like Made in China 2025 emphasize innovation in pharmaceutical manufacturing, creating opportunities for Pharma 4.0 technologies.
- India’s Focus on Automation: Under the Pharma Vision 2020, India is boosting automation in manufacturing to enhance global competitiveness and ensure regulatory compliance.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2449
Latin America
- Digital Healthcare Investments: Countries like Brazil and Mexico are investing in digital healthcare infrastructure, indirectly promoting the adoption of Pharma 4.0 for seamless integration of supply chains and regulatory processes.
Impact of Key Player Initiatives and Partnerships on the Pharma 4.0 Market
The Pharma 4.0 landscape is shaped significantly by strategic collaborations, mergers, and technological innovations undertaken by key players.
Strategic Partnerships
- Joint Development of IoT Solutions: Siemens and GEA Group have partnered to develop IoT-enabled systems for pharmaceutical production, enhancing real-time monitoring capabilities.
- Blockchain for Traceability: IBM’s collaboration with Merck focuses on blockchain applications for drug traceability, ensuring regulatory compliance and supply chain transparency.
Mergers and Acquisitions
- Expanding Market Presence: Rockwell Automation’s acquisition of Avnet Data Security enhances its portfolio of Pharma 4.0 solutions, focusing on cybersecurity for automated manufacturing systems.
- Innovation Synergies: Schneider Electric’s partnership with AVEVA brings advanced analytics and AI-driven insights to pharmaceutical manufacturing.
Technology Advancements
- AI and Machine Learning Integration: Companies like Pfizer are leveraging AI to optimize manufacturing processes and predict potential production issues, ensuring efficiency and compliance.
- IoT-Enabled Smart Equipment: ABB’s smart sensors and equipment enable real-time monitoring of production environments, driving efficiency in Pharma 4.0 ecosystems.
What Role Do Sustainability and Compliance Play in Pharma 4.0?
Sustainability and regulatory compliance are critical drivers for the Pharma 4.0 market.
Sustainability Goals
- Energy-Efficient Operations: Companies are integrating energy-saving technologies to reduce carbon footprints, aligning with global sustainability goals.
- Waste Reduction: AI-driven predictive maintenance systems reduce waste in production processes, contributing to greener manufacturing practices.
Regulatory Compliance
- Real-Time Quality Control: Pharma 4.0 enables continuous monitoring and real-time analytics to ensure compliance with stringent regulatory standards like FDA’s cGMP and EMA’s guidelines.
- Data Integrity: Blockchain solutions ensure data security and integrity, critical for meeting global regulatory requirements.
Competitive Landscape of the Pharma 4.0 Market
The Pharma 4.0 market is characterized by intense competition, with companies striving to enhance their technological offerings and expand their global footprint.
Key Market Players
- ABB Ltd.
- Siemens AG
- Rockwell Automation
- Schneider Electric
- Honeywell International
Innovation and Market Reach
- Global Expansion: Key players are targeting emerging markets like India and Southeast Asia to leverage untapped growth opportunities.
- Focus on Customization: Tailored solutions for specific pharmaceutical needs, such as personalized medicine manufacturing, are gaining traction.