Continuous Processing Lines for Pharma Market Size, Trends, and Forecast 2024–2034
Continuous Processing Lines for Pharma Market Size
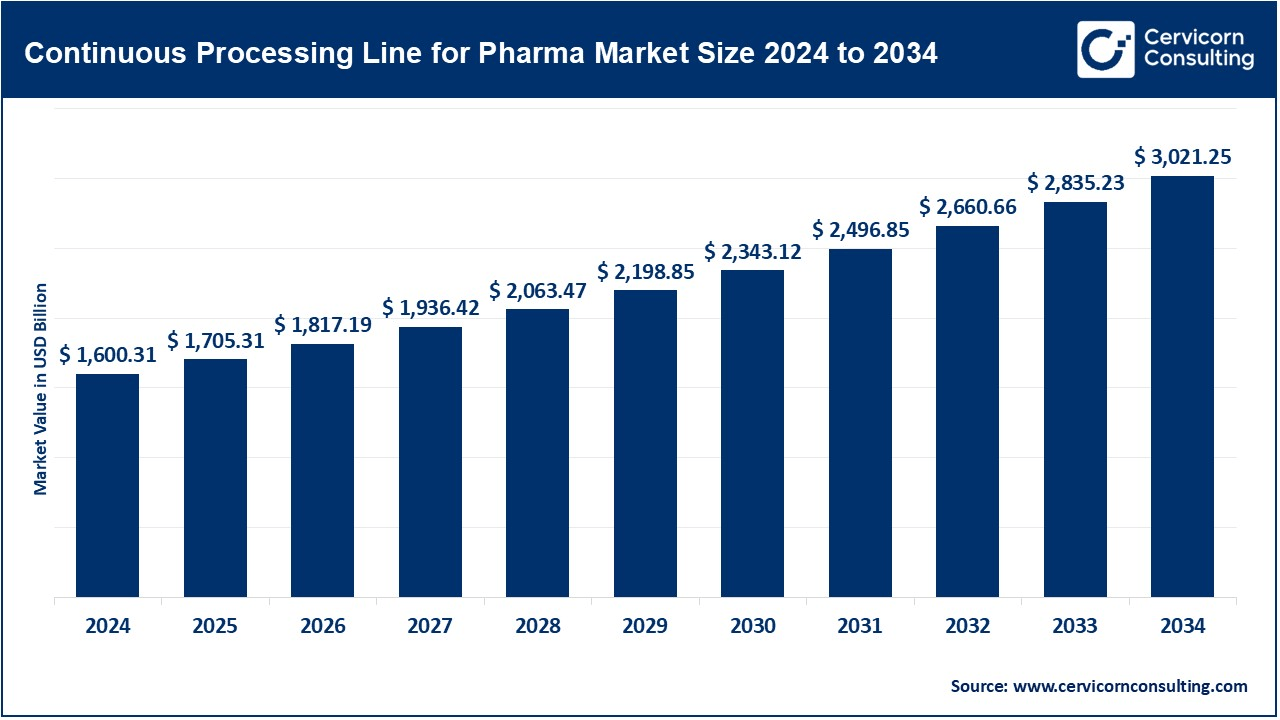
The global continuous processing lines for pharma market was worth USD 1,600.31 billion in 2024 and is anticipated to expand to around USD 3,021.25 billion by 2034, registering a compound annual growth rate (CAGR) of 6.56% from 2025 to 2034.
Understanding Continuous Processing Lines for Pharma Market
Continuous processing in the pharmaceutical industry refers to the uninterrupted production of drugs, where raw materials are continuously fed into the system, and finished products are consistently produced. Unlike traditional batch processing, which involves discrete steps and pauses between stages, continuous processing integrates all manufacturing stages into a seamless flow. This approach enhances efficiency, reduces production time, and minimizes human intervention, leading to improved product quality and consistency.
Importance of Continuous Processing
The adoption of continuous processing is pivotal for several reasons:
- Efficiency and Speed: Continuous systems can reduce production times from weeks to mere hours, enabling faster delivery of medications to the market.
- Quality Control: Real-time monitoring ensures consistent product quality, reducing the risk of deviations and enhancing patient safety.
- Cost Reduction: By minimizing waste and optimizing resource utilization, continuous processing can lead to significant cost savings.
- Regulatory Support: Agencies like the FDA and EMA advocate for continuous manufacturing due to its potential to improve drug quality and supply chain robustness.
Continuous Processing Lines for Pharma Market Growth Factors
The continuous processing market in pharmaceuticals is experiencing robust growth, driven by several key factors:
- Technological Advancements: Innovations in automation, AI, and real-time analytics have made continuous processing more feasible and effective.
- Regulatory Encouragement: Regulatory bodies are actively promoting continuous manufacturing to enhance drug quality and supply chain resilience.
- Demand for Personalized Medicine: The shift towards personalized therapies necessitates flexible and efficient manufacturing processes, which continuous systems can provide.
- Sustainability Goals: Continuous processing aligns with environmental objectives by reducing waste and energy consumption.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2613
Continuous Processing Lines for Pharma Market Leading Companies
ACG Engineering
- Specialization: Offers comprehensive solutions for solid dosage manufacturing, including granulation, capsule filling, tablet pressing, coating, and packaging.
- Key Focus Areas: Integration of machinery with digital technologies for enhanced efficiency and compliance.
- Notable Features: Over 20,000 machine installations globally, reflecting a strong market presence.
- Market Share: Significant presence in Asia and expanding globally.
- Global Presence: Operations in over 100 countries across six continents.
Antares Vision Group
- Specialization: Provides inspection systems, track & trace solutions, and smart data management for the pharmaceutical industry.
- Key Focus Areas: Ensuring product integrity and compliance through advanced serialization and traceability technologies.
- Notable Features: Leader in anti-counterfeiting measures and supply chain transparency.
- Market Share: Strong presence in Europe and expanding in North America and Asia.
- Global Presence: Operates in over 60 countries worldwide.
BOSCH Packaging Technology
- Specialization: Offers a wide range of packaging and processing solutions for the pharmaceutical industry.
- Key Focus Areas: Development of continuous manufacturing systems and integration of digital technologies.
- Notable Features: Known for high-quality engineering and innovation in packaging solutions.
- Market Share: Significant global market presence.
- Global Presence: Operations in over 150 countries.
Bühler Group
- Specialization: Provides processing equipment and services for various industries, including pharmaceuticals.
- Key Focus Areas: Development of continuous processing technologies and sustainable solutions.
- Notable Features: Strong emphasis on innovation and sustainability.
- 2024 Revenue (approx): CHF 3.0 billion.
- Market Share: Robust presence in Europe and expanding globally.
- Global Presence: Operations in over 140 countries.
Fette Compacting
- Specialization: Focuses on tablet press machines and capsule filling equipment for the pharmaceutical industry.
- Key Focus Areas: Enhancing efficiency and precision in solid dosage manufacturing.
- Notable Features: Pioneer in tablet compression technology with a strong R&D focus.
- 2024 Revenue (approx): €370 million (2020 data).
- Market Share: Leading position in Europe and growing presence in Asia and North America.
- Global Presence: Operations in over 50 countries.
Continuous Processing Lines for Pharma Market Leading Trends and Their Impact
- Modular Manufacturing Units: The development of Portable, Continuous, Miniature, and Modular (PCMM) systems allows for flexible and scalable production, enabling rapid response to market demands.
- Integration of AI and Automation: Advanced analytics and machine learning enhance process control, predictive maintenance, and quality assurance.
- Sustainability Initiatives: Continuous processing aligns with environmental goals by reducing energy consumption and waste, contributing to greener manufacturing practices.
- Regulatory Evolution: Regulatory bodies are updating guidelines to accommodate and encourage continuous manufacturing, facilitating smoother adoption by the industry.
Successful Global Implementations
- Vertex Pharmaceuticals: Achieved FDA approval for Orkambi, produced using continuous manufacturing, marking a significant milestone in the industry.
- Eli Lilly: Established a continuous manufacturing line in Kinsale, Ireland, for the production of prexasertib, demonstrating the feasibility of continuous API synthesis.
- Pfizer and GSK: Collaborated on the development of PCMM systems, showcasing the potential of modular continuous manufacturing units.
Continuous Processing Lines for Pharma Market Regional Analysis and Government Initiatives
- India: The Hyderabad Pharma City project aims to create a hub for pharmaceutical manufacturing, including continuous processing facilities, with significant government investment and support.
- United Kingdom: The North East of England Process Industry Cluster (NEPIC) promotes innovation and investment in continuous manufacturing, supported by government grants and initiatives.
- United States: The FDA actively encourages the adoption of continuous manufacturing through guidance documents and regulatory support, fostering innovation in the pharmaceutical sector.
- Asia-Pacific: Countries like China and Japan are investing in continuous manufacturing technologies to enhance pharmaceutical production capabilities and meet growing healthcare demands.
The global shift towards continuous processing in pharmaceuticals is driven by technological advancements, regulatory support, and the need for efficient, high-quality drug production. As the industry continues to evolve, continuous manufacturing stands at the forefront of this transformation, offering solutions that meet the demands of modern healthcare.
To Get Detailed Overview, Contact Us: https://www.cervicornconsulting.com/contact-us
Read Report: U.S. Corporate Wellness Market Outlook 2024–2034: Growth, Innovations, and Policy Impact