Cell and Gene Therapy Market Trends, Growth & Insights 2025-2034
Cell and Gene Therapy Market Overview
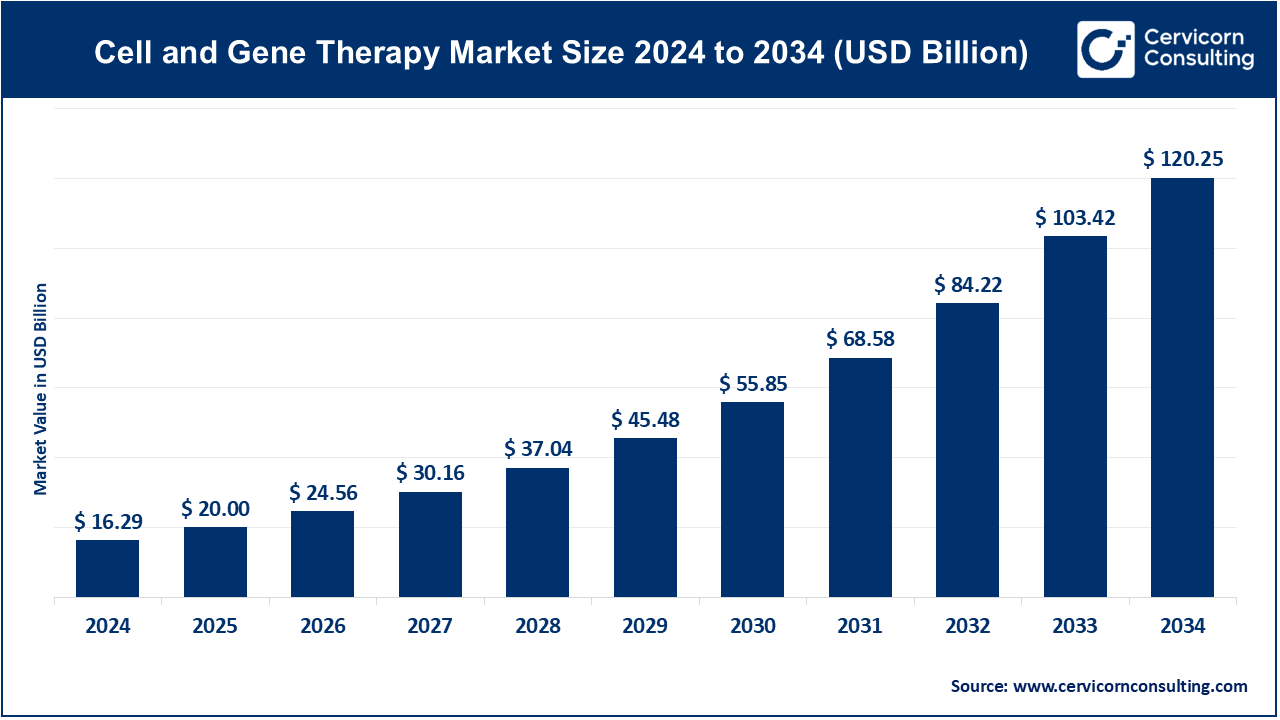
The global cell and gene therapy market was valued at an estimated USD 16.29 billion in 2024 and is expected to increase to USD 120.25 billion by 2034, with a compound annual growth rate (CAGR) of 22.8%.
The global cell and gene therapy market is experiencing unprecedented growth due to advancements in biotechnology, increasing prevalence of chronic diseases, rising investments in research and development, and supportive regulatory frameworks. The demand for personalized medicine, combined with breakthroughs in gene editing technologies like CRISPR-Cas9, and the growing approval rates of innovative therapies are also key contributors to this growth trajectory.
What is the Cell and Gene Therapy Market?
The cell and gene therapy market encompasses the development, manufacturing, and commercialization of treatments that use cells and genetic material to address diseases. These therapies represent a cutting-edge branch of regenerative medicine, focusing on:
- Cell Therapy: Involves the introduction of living cells into a patient to treat or cure diseases. Examples include:
- Stem Cell Therapies: Used for conditions like hematological disorders and organ repair.
- CAR-T Cell Therapies: Target specific cancer cells.
- Gene Therapy: Involves the introduction, alteration, or removal of genetic material to correct or mitigate genetic disorders. Examples include:
- Treatments for rare inherited diseases like spinal muscular atrophy.
- Cancer immunotherapies targeting mutations.
The market includes stakeholders such as biotechnology companies, pharmaceutical firms, research institutions, and healthcare providers engaged in the innovation, production, and delivery of these therapies.
Why is the Cell and Gene Therapy Market Important?
The significance of the cell and gene therapy market lies in its potential to revolutionize healthcare by addressing previously untreatable or hard-to-treat conditions. Key reasons for its importance include:
- Unmet Medical Needs:
- Many rare genetic diseases and advanced-stage cancers lack effective treatments. Cell and gene therapies provide novel solutions to these challenges.
- Personalized Medicine:
- These therapies can be tailored to the specific needs of individual patients, ensuring higher efficacy and fewer side effects.
- Long-Term Benefits:
- Many cell and gene therapies aim for curative outcomes, potentially eliminating the need for chronic treatments and reducing healthcare costs over time.
- Technological Advancements:
- Innovations in biotechnology, such as CRISPR and next-generation sequencing, have accelerated the development of these therapies, making them more accessible and effective.
Top Companies in the Global Cell and Gene Therapy Market
Below is an analysis of leading companies shaping the cell and gene therapy market, focusing on their specializations, key focus areas, notable features, 2023 revenue, market share, and global presence.
Novartis International AG
- Specialization: Pioneering CAR-T cell therapies and gene-editing technologies.
- Key Focus Areas: Oncology, ophthalmology, and rare genetic disorders.
- Notable Features: Developer of Kymriah®, the first FDA-approved CAR-T cell therapy.
- 2024 Revenue (approx.): $54 billion (total revenue).
- Market Share (approx.): 12% in the cell and gene therapy sector.
- Global Presence: Strong presence across North America, Europe, and Asia-Pacific.
Gilead Sciences, Inc.
- Specialization: Cell-based immunotherapies.
- Key Focus Areas: Oncology and HIV/AIDS treatments.
- Notable Features: Acquired Kite Pharma to strengthen its CAR-T therapy portfolio, including Yescarta®.
- 2024 Revenue (approx.): $28 billion.
- Market Share (approx.): 10% in the cell therapy segment.
- Global Presence: Operations spanning over 35 countries.
Bristol-Myers Squibb Company
- Specialization: Advanced immuno-oncology and cell therapy.
- Key Focus Areas: Hematologic malignancies and solid tumors.
- Notable Features: Breyanzi®, a leading CAR-T cell therapy for lymphoma.
- 2024 Revenue (approx.): $46 billion.
- Market Share (approx.): 15% in the CAR-T therapy segment.
- Global Presence: Global operations with a strong focus in the Americas, Europe, and Asia.
bluebird bio, Inc.
- Specialization: Gene therapies targeting rare diseases.
- Key Focus Areas: Beta-thalassemia, sickle cell disease, and cerebral adrenoleukodystrophy.
- Notable Features: Developer of Zynteglo® and Skysona®, breakthrough gene therapies.
- 2024 Revenue (approx.): $180 million.
- Market Share (approx.): 4% in the gene therapy market.
- Global Presence: North America and Europe.
Spark Therapeutics, Inc. (a Roche company)
- Specialization: Genetic therapies for inherited retinal diseases.
- Key Focus Areas: Ophthalmology and hemophilia.
- Notable Features: Luxturna®, the first FDA-approved gene therapy for a genetic disease.
- 2024 Revenue (approx.): $700 million.
- Market Share (approx.): 5% in the gene therapy sector.
- Global Presence: North America, Europe, and selected Asia-Pacific markets.
Leading Trends and Their Impact
- Personalized Medicine: Personalized therapies are revolutionizing patient care by targeting specific genetic markers, improving treatment outcomes, and minimizing side effects.
- Impact: Enhanced patient satisfaction and adoption of innovative treatments.
- Advances in Gene Editing Technologies: CRISPR and similar tools are enabling precise genetic modifications, expanding therapeutic possibilities.
- Impact: Increased pipeline diversity and accelerated drug development timelines.
- Collaborative R&D Ecosystems: Partnerships between biotech firms, academic institutions, and pharmaceutical giants are driving innovation.
- Impact: Reduced development costs and shared technological advancements.
- Regulatory Support: Expedited approval pathways and incentives for orphan drugs are fostering market growth.
- Impact: Quicker market entry and increased investment in rare disease treatments.
- Automation in Manufacturing: Adoption of automated processes is improving production efficiency and scalability.
- Impact: Reduced costs and consistent product quality.
Successful Examples of Cell and Gene Therapy
- Kymriah® (Novartis): The first CAR-T cell therapy approved by the FDA, Kymriah has achieved notable success in treating pediatric and young adult patients with acute lymphoblastic leukemia (ALL).
- Zolgensma® (Novartis): A one-time gene therapy for spinal muscular atrophy, Zolgensma has transformed patient outcomes with its groundbreaking approach.
- Luxturna® (Spark Therapeutics): Addressing inherited retinal dystrophy caused by RPE65 mutations, Luxturna restores functional vision in eligible patients.
Regional Analysis: Government Initiatives and Policies Shaping the Market
North America
- Key Factors: Dominance in R&D, strong regulatory support, and significant funding from both public and private sectors.
- Government Initiatives: The FDA’s regenerative medicine advanced therapy (RMAT) designation and substantial NIH grants boost innovation.
Europe
- Key Factors: Robust academic-industry collaborations and a supportive regulatory environment.
- Government Initiatives: The European Medicines Agency (EMA) offers PRIME (PRIority MEdicines) designation for accelerated approval of promising therapies.
Asia-Pacific
- Key Factors: Growing investment in biotechnology, expanding healthcare infrastructure, and increasing prevalence of chronic diseases.
- Government Initiatives: China’s support for CAR-T therapies and Japan’s Sakigake designation for fast-tracking innovative treatments.
Latin America
- Key Factors: Rising awareness of advanced therapies and increasing healthcare expenditure.
- Government Initiatives: Collaborative programs aimed at boosting local biotech capabilities.
Middle East and Africa
- Key Factors: Growing medical tourism and increasing adoption of advanced healthcare technologies.
- Government Initiatives: Investments in state-of-the-art healthcare infrastructure and partnerships with global biotech firms.
To Get Detailed Overview, Contact Us: https://www.cervicornconsulting.com/contact-us
Read Report: Artificial Intelligence (AI) in Healthcare Market Key Growth Factors and Industry Insights by 2033